

Many other rare types of decay, such as spontaneous fission or neutron emission are known. Unstable isotopes decay through various radioactive decay pathways, most commonly alpha decay, beta decay, or electron capture. If there are too many or too few neutrons for a given number of protons, the resulting nucleus is not stable and it undergoes radioactive decay. As a result, as the number of protons increases, an increasing ratio of neutrons to protons is needed to form a stable nucleus. Neutrons stabilize the nucleus, because they attract each other and protons, which helps offset the electrical repulsion between protons. There are only certain combinations of neutrons and protons, which forms stable nuclei. These two forces compete, leading to various stability of nuclei. The neutron has a mean square radius of about 0.8×10−15 m, or 0.8 fm, and it is a spin-½ fermion.Ītomic nuclei consist of protons and neutrons, which attract each other through the nuclear force, while protons repel each other via the electric force due to their positive charge. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg-marginally greater than that of the proton but nearly 1839 times greater than that of the electron. In the universe, neutrons are abundant, making up more than half of all visible matter.
#Atomic number of magnesium free#
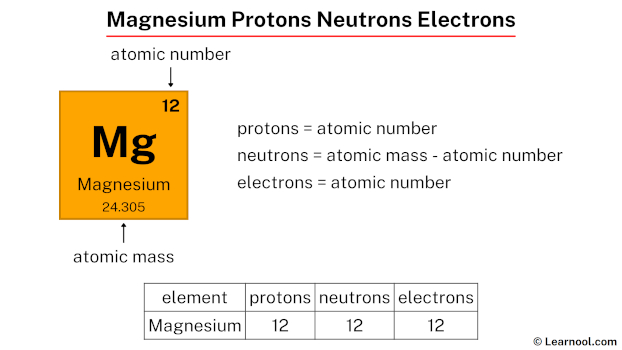
The free metal burns with a characteristic brilliant-white light.Ī neutron is one of the subatomic particles that make up matter. The free element (metal) can be produced artificially, and is highly reactive (though in the atmosphere, it is soon coated in a thin layer of oxide that partly inhibits reactivity). Magnesium occurs naturally only in combination with other elements, where it invariably has a +2 oxidation state. In the periodic table, the elements are listed in order of increasing atomic number Z.Įlectron configuration of Magnesium is 3s2. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. The configuration of these electrons follows from the principles of quantum mechanics. Since the number of electrons and their arrangement are responsible for the chemical behavior of atoms, the atomic number identifies the various chemical elements. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom. Therefore, the number of electrons in neutral atom of Magnesium is 12. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Magnesium-26 is composed of 12 protons, 12 neutrons, and 12 electrons.
Magnesium-25 is composed of 12 protons, 13 neutrons, and 12 electrons. Magnesium-24 is composed of 12 protons, 12 neutrons, and 12 electrons. The lighter isotopes mostly decay to isotopes of sodium while the heavier isotopes decay to isotopes of aluminium. The longest-lived radioisotope is 28Mg with a half-life of 20.915 hours. Magnesium naturally occurs in three stable isotopes, 24Mg, 25Mg, and 26Mg. Mass numbers of typical isotopes of Magnesium are 24 25 26. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z.įor stable elements, there is usually a variety of stable isotopes. Neutron number plus atomic number equals atomic mass number: N+Z=A.

The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10 -19 coulombs. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus.


 0 kommentar(er)
0 kommentar(er)
